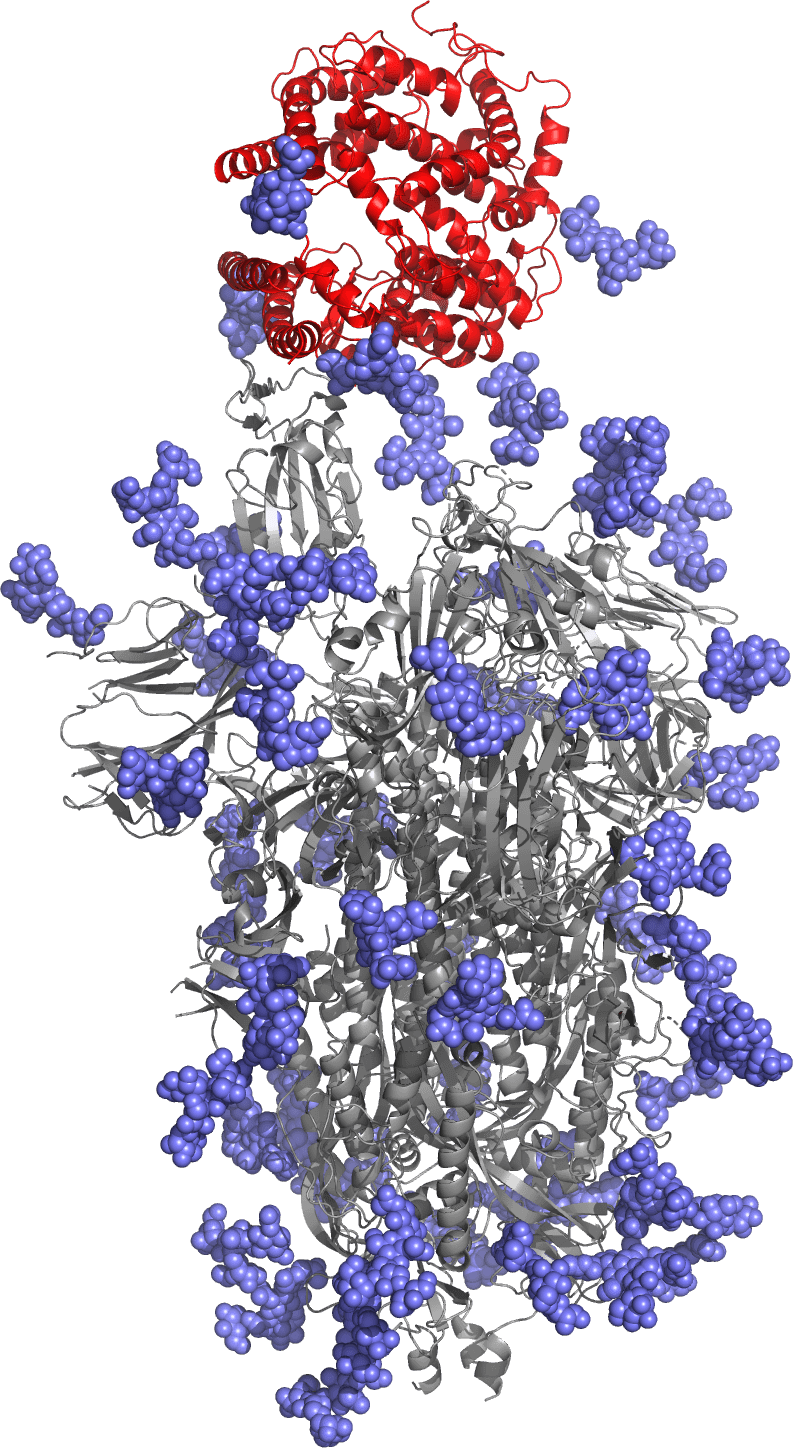
The SARS-CoV-2 spike protein in grey with the host receptor in red. The Phylex antigen is made of regions of the spike protein close to the receptor. Natural sugars carried by the protein are in blue.
The head domain of the Nipah virus G attachment glycoprotein is decorated with four natural sugars. This 3-dimensional structure was predicted using the AI deep learning system AlphaFold2. Demis Hassabis and John Jumper received the 2024 Nobel Prize in chemistry for the development of AlphaFold2. The Phylex vaccine nanoparticle displays 60 copies of this antigen.
AI DESIGN OF ANTIGENS
Recent progress in computational analysis and artificial intelligence deep learning allows us to design antigens that elicit specific classes of neutralizing antibodies, the primary defense against viral infection. These neutralizing antibodies prevent virus entry into the host cell.
For our SARS-CoV-2 vaccine, the Phylex antigen is based on regions of the viral spike protein close to the host receptor. The vaccine is designed to protect against the latest XEC and KP.3 variants of the SARS-CoV-2 coronavirus, all with increased transmissibility and resistance to current vaccines. It is a complement vaccine, designed to elicit a different class of neutralizing antibodies as compared with current vaccines and to address the major issue of immune imprinting.
For our Nipah vaccine the antigen is based upon the head domain of the G attachment protein of the Nipah virus, and displays most epitopes of neutralizing antibodies.
All our vaccines are nanoparticles that induce long-lasting immunity and enhance protection against viral dissemination and neuropathology associated with viral infection, such as long COVID syndrome and Nipah encephalitis.
Recent progress in computational analysis and artificial intelligence deep learning allows us to design antigens that elicit specific classes of neutralizing antibodies, the primary defense against viral infection. These neutralizing antibodies prevent virus entry into the host cell.
For our SARS-CoV-2 vaccine, the Phylex antigen is based on regions of the viral spike protein close to the host receptor. The vaccine is designed to protect against the latest XEC and KP.3 variants of the SARS-CoV-2 coronavirus, all with increased transmissibility and resistance to current vaccines. It is a complement vaccine, designed to elicit a different class of neutralizing antibodies as compared with current vaccines and to address the major issue of immune imprinting.
For our Nipah vaccine the antigen is based upon the head domain of the G attachment protein of the Nipah virus, and displays most epitopes of neutralizing antibodies.
All our vaccines are nanoparticles that induce long-lasting immunity and enhance protection against viral dissemination and neuropathology associated with viral infection, such as long COVID syndrome and Nipah encephalitis.
mRNA NANOPARTICLE
We have developed a flexible mRNA nanoparticle platform for the rapid development of our vaccine candidates. A messenger RNA directly encodes for the self-assembling nanoparticle. This highly immunogenic nanoparticle displays 60 copies of the antigen and has a size, geometry and symmetry mimicking the virus.
Proof-of-principle studies in collaboration with the Pasteur Institute showed that our mRNA nanoparticle vaccine elicits a robust immune response with neutralizing antibodies and protective immunity against the delta variant of SARS-CoV-2:
A mRNA vaccine encoding for a RBD 60-mer nanoparticle elicits neutralizing antibodies and protective immunity against the SARS-CoV-2 delta variant in transgenic K18-hACE2 mice
Frontiers in Immunology original research article published on July 6, 2022
A recent study in collaboration with scientists of the U.S. Centers for Disease Control and Prevention (CDC) demonstrated that our mRNA nanoparticle vaccine elicits a robust neutralizing antibodies response against the Nipah virus with neutralization titers 3-fold the average titers of individuals who survived a Nipah infection in Bangladesh:
A mRNA vaccine encoding for a 60-mer G glycoprotein nanoparticle elicits a robust neutralizing antibodies response against the Nipah virus
We have developed a flexible mRNA nanoparticle platform for the rapid development of our vaccine candidates. A messenger RNA directly encodes for the self-assembling nanoparticle. This highly immunogenic nanoparticle displays 60 copies of the antigen and has a size, geometry and symmetry mimicking the virus.
Proof-of-principle studies in collaboration with the Pasteur Institute showed that our mRNA nanoparticle vaccine elicits a robust immune response with neutralizing antibodies and protective immunity against the delta variant of SARS-CoV-2:
A mRNA vaccine encoding for a RBD 60-mer nanoparticle elicits neutralizing antibodies and protective immunity against the SARS-CoV-2 delta variant in transgenic K18-hACE2 mice
Frontiers in Immunology original research article published on July 6, 2022
A recent study in collaboration with scientists of the U.S. Centers for Disease Control and Prevention (CDC) demonstrated that our mRNA nanoparticle vaccine elicits a robust neutralizing antibodies response against the Nipah virus with neutralization titers 3-fold the average titers of individuals who survived a Nipah infection in Bangladesh:
A mRNA vaccine encoding for a 60-mer G glycoprotein nanoparticle elicits a robust neutralizing antibodies response against the Nipah virus
COUNTERING IMMUNE IMPRINTING
A major issue with all current COVID-19 vaccines, immune imprinting describes a narrow immune memory, limited to the wild type SARS-CoV-2 virus. Immune imprinting is caused by initial infection, as well as first and second generation vaccines and breakthrough infections. All vaccines to date contain epitopes in the spike protein common to the wild type SARS-CoV-2 virus that are preferentially recognized by the limited immune memory and elicit a narrow immune response. Our vaccine elicits a different class of neutralizing antibodies that complements and broadens the immune response.
LONG-LASTING IMMUNITY
Bone-marrow long lived plasma cells (BM LLPCs) are white blood cells critical for a durable immune response. It has been recently demonstrated that current COVID-19 mRNA vaccines fail to imprint BM LLPCs and therefore induce rapidly waning antibodies. Our nanoparticle vaccines with optimal repetitive antigen spacing achieve proper activation and generation of BM LLPCs, resulting in long-lasting immunity.
A major issue with all current COVID-19 vaccines, immune imprinting describes a narrow immune memory, limited to the wild type SARS-CoV-2 virus. Immune imprinting is caused by initial infection, as well as first and second generation vaccines and breakthrough infections. All vaccines to date contain epitopes in the spike protein common to the wild type SARS-CoV-2 virus that are preferentially recognized by the limited immune memory and elicit a narrow immune response. Our vaccine elicits a different class of neutralizing antibodies that complements and broadens the immune response.
LONG-LASTING IMMUNITY
Bone-marrow long lived plasma cells (BM LLPCs) are white blood cells critical for a durable immune response. It has been recently demonstrated that current COVID-19 mRNA vaccines fail to imprint BM LLPCs and therefore induce rapidly waning antibodies. Our nanoparticle vaccines with optimal repetitive antigen spacing achieve proper activation and generation of BM LLPCs, resulting in long-lasting immunity.
